
Atom illustration, Bohr model Sodium Atom Chemistry Rutherford model
Figure \(\PageIndex{1}\): A Bhor's model can be used to diagram the location of electrons in each energy shell for an atom. Notice that protons go in the nucleus of the atom and electrons are drawn on orbits surrounding the nucleus. Image from Wikimedia commons. Example \(\PageIndex{2}\) Draw the Bohr's model for sodium (Na).
.PNG)
Bohr Models and Lewis Dot Diagrams Presentation Chemistry
Immediately before 1913, the Rutherford model conceived of an atom as consisting of a tiny positively charged heavy core, called a nucleus, surrounded by light, planetary negative electrons revolving in circular orbits of arbitrary radii. Britannica Quiz Matter and More Quiz How does Niels Bohr's atomic model work?
Bohr Model Of Sodium
which is identical to the Rydberg equation in which R ∞ = k h c. R ∞ = k h c. When Bohr calculated his theoretical value for the Rydberg constant, R ∞, R ∞, and compared it with the experimentally accepted value, he got excellent agreement. Since the Rydberg constant was one of the most precisely measured constants at that time, this level of agreement was astonishing and meant that.

Sodium Bohr Model — Diagram, Steps To Draw Techiescientist
The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Only certain electron orbits are permitted.

Structure of sodium atom Modelos atômicos, Modelos
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.

Sodium Bohr model
The electron cloud model An electron cloud model of a helium-4 atom is shown below. [What do the scales mean on this model?] In this model, the black "cloud" represents the volume of space where electrons are likely to be found. The darker the region, the more likely electrons are to be found there.
Sodium (Na) AMERICAN ELEMENTS
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ).
.PNG)
Bohr Model Of Sodium
Bohr's Model of an Atom was proposed in 1913 as a modification of the prevailing Saturnian model developed by Bohr's mentor Ernest Rutherford, and the Bohr model is sometimes referred to.
Sodium (Na) AMERICAN ELEMENTS
Thanks Erik T. for being so awesome at chemistry and willing to teach us how to build a sodium atom.-----Thanks for watching and please subscribe for more sc.

ScIU Conversations in Science at Indiana University
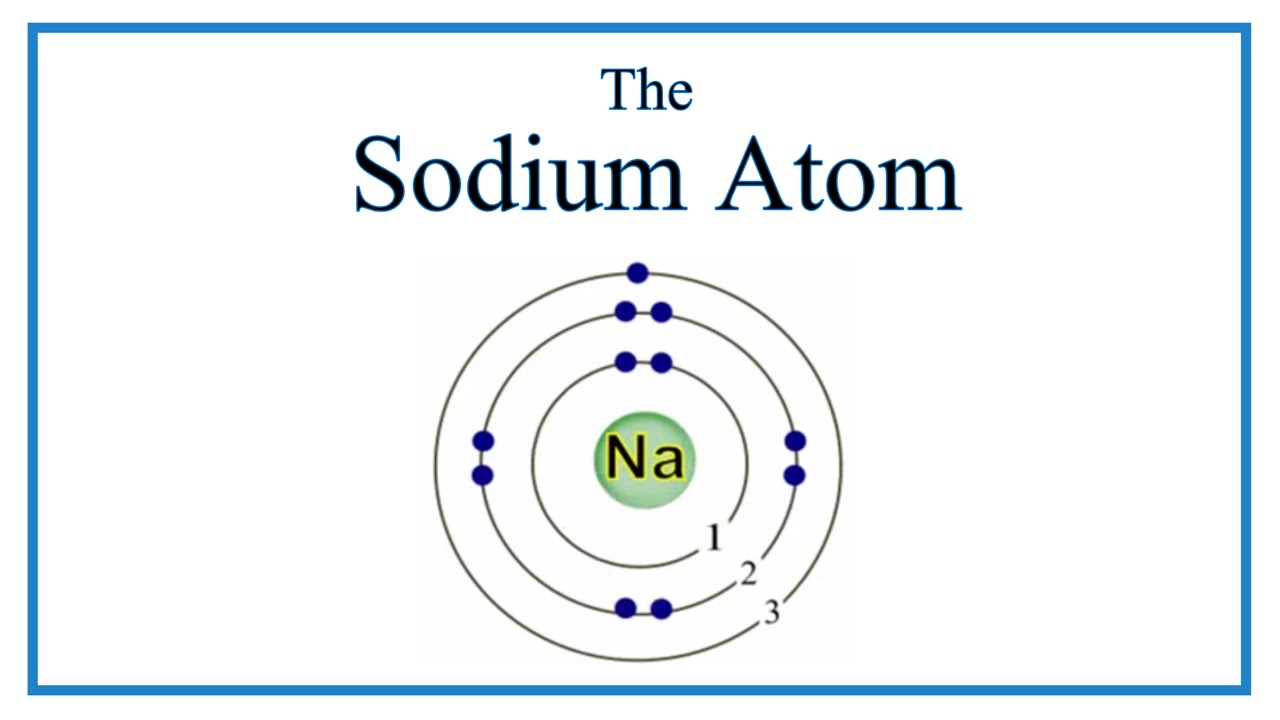
The figure seen above illustrates Bohr's theories applied to the sodium atom. Note how the Bohr model, like that of Lewis, assumes a shell structure. There are two electrons in the innermost shell, eight electrons in the next shell, and a single electron in the outermost shell. Like Lewis' model, Bohr's model was only partially successful.
Sodium atom Bohr model stock vector. Illustration of science 267662272
Figure 7.3.2 7.3. 2: The emission spectra of sodium and mercury. Sodium and mercury spectra. Many street lights use bulbs that contain sodium or mercury vapor. Due to the very different emission spectra of these elements, they emit light of different colors. The lines in the sodium lamp are broadened by collisions.
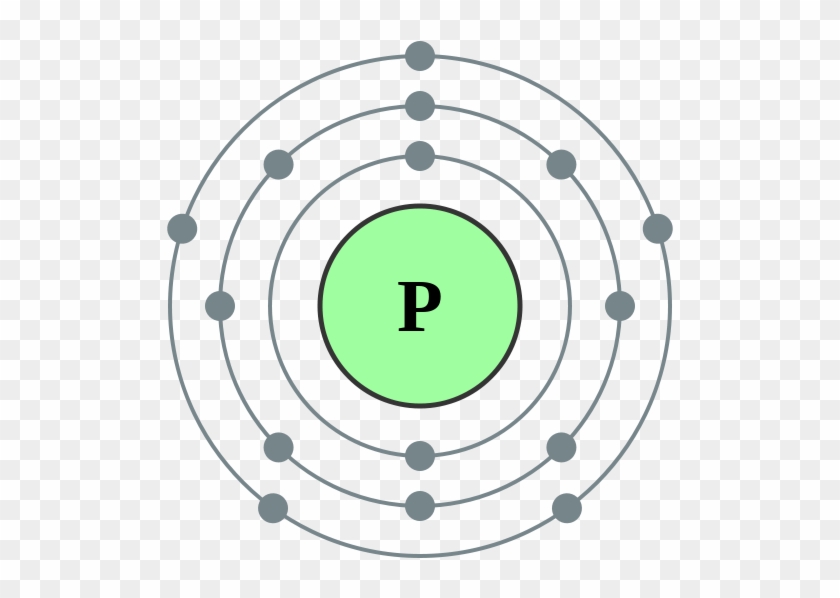
Bohr Model Of A Phosphorus Atom Electron Configuration Of Sodium
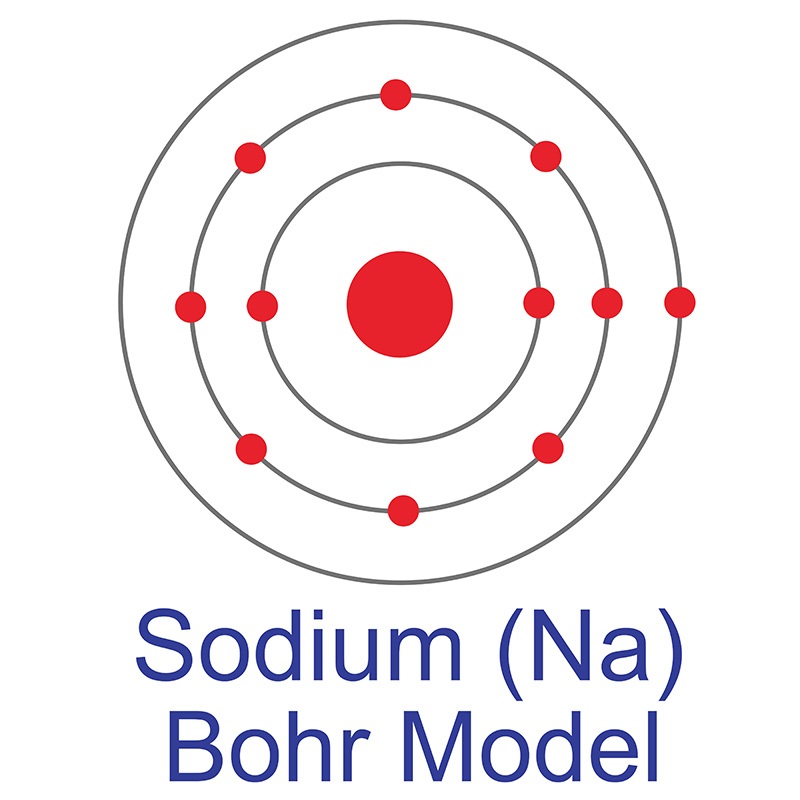
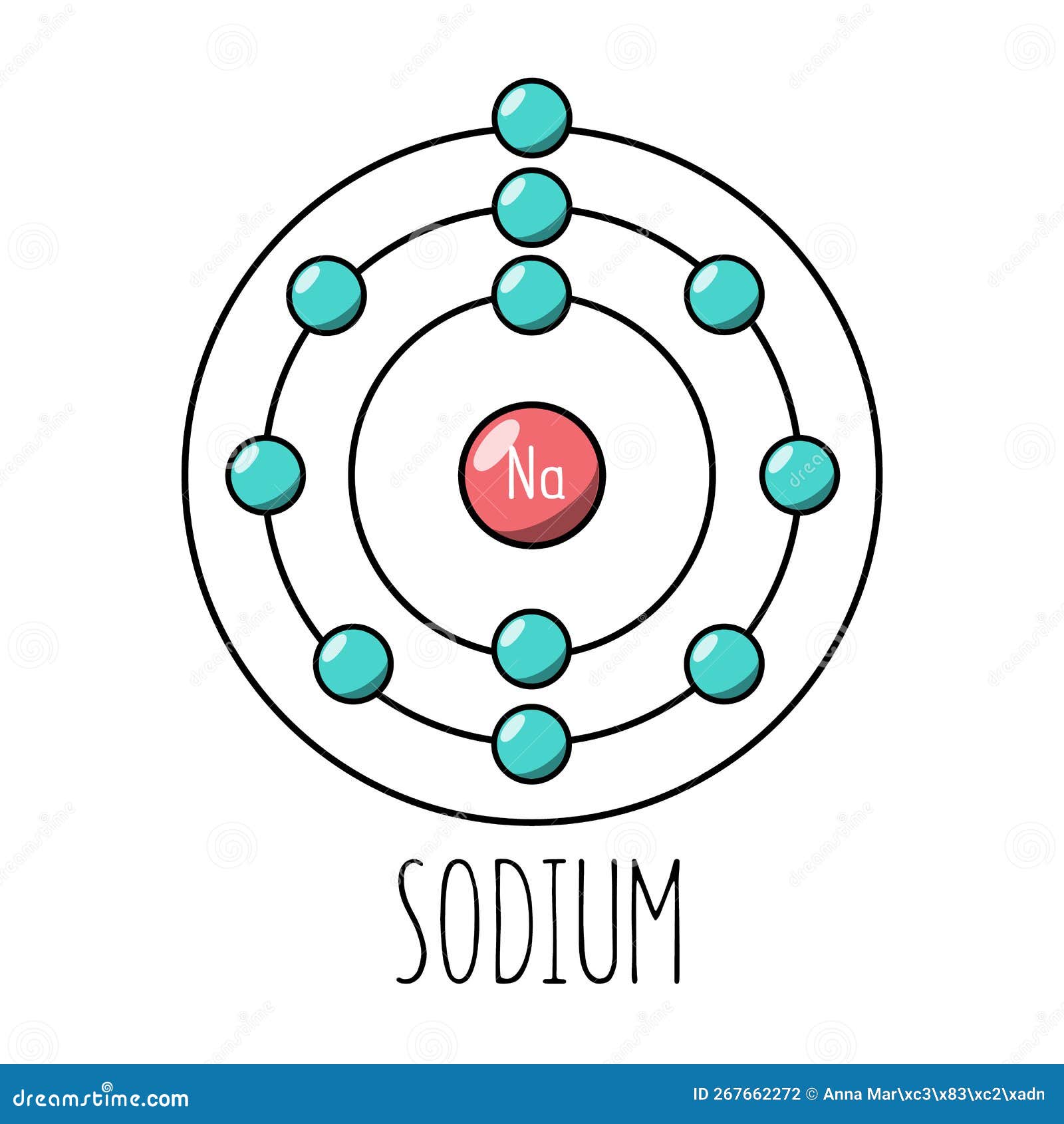
The Bohr Model of Sodium (Na) has a nucleus that contains 12 neutrons and 11 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Sodium contains only 1 electron that also called valence electron. Page Contents show How to draw Bohr Model of Sodium (Na)?

Bohr Diagram Of Sodium Atom model, Atom diagram, Atom model project
Creating a sodium Bohr diagram is a way to visually represent the arrangement of electrons in a sodium atom based on the Bohr model. The Bohr model suggests that electrons occupy specific energy levels or shells around the nucleus of an atom. Each shell can hold a certain number of electrons, and the diagram helps to show the distribution of.

Figure \ Electron Shell Configuration Of Sodium 1200x1200 PNG
Sodium(Na) electron configuration (Bohr model) Electron configuration through orbitals follows different principles. For example Aufbau principle, Hund's principle, and Pauli's exclusion principle. Sodium electron configuration through orbit. Scientist Niels Bohr was the first to give an idea of the atom's orbit. He provided a model of.
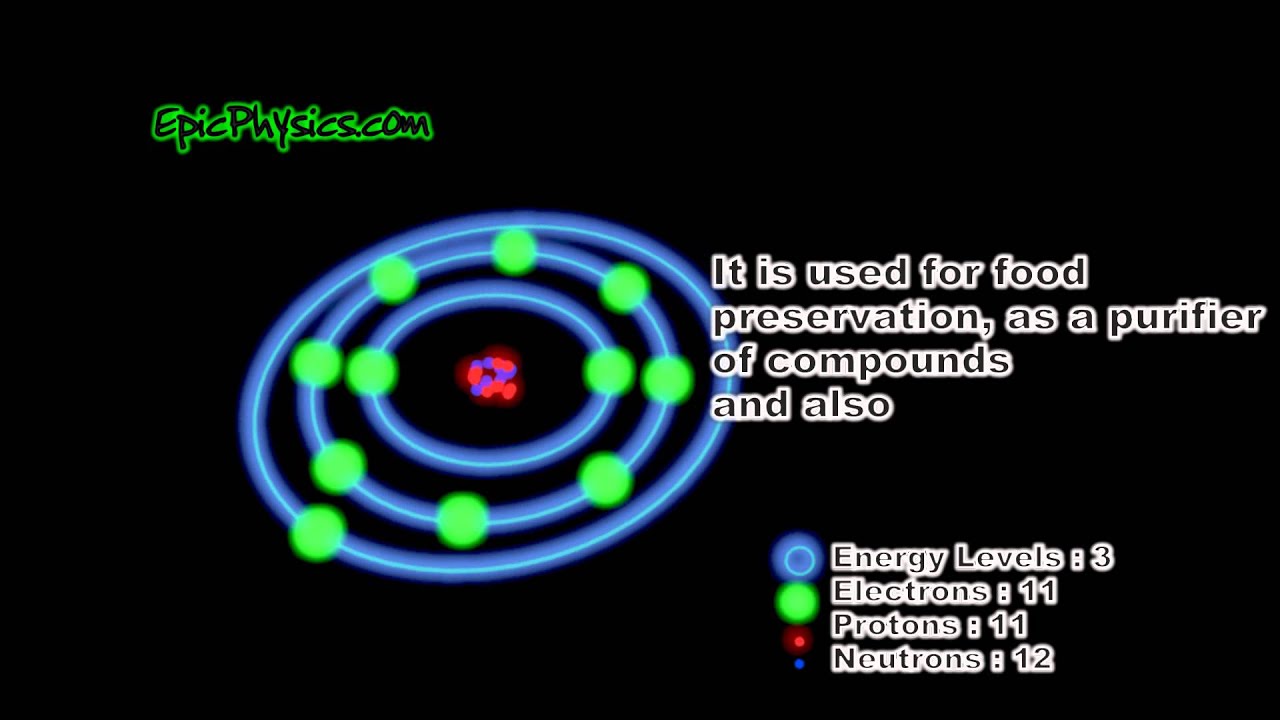
Sodium Facts & Bohr Model YouTube
The Bohr model of sodium contains a nucleus having 11 protons and 12 neutrons in the center, and around this nucleus, there are three electron shells containing 11 electrons. Atomic Structure of the Sodium Atom (Na) Watch on Contents Steps #1 Write protons, neutrons, and electrons of sodium atom #2 Draw nucleus of sodium atom
Bohr Model Drawing Oxygen at Explore collection of
Sodium Bohr Model — Diagram, Steps To Draw Sodium is a highly reactive metal element. It has the atomic number 11 and is represented by the symbol Na. It belongs to group 1A of the periodic table and hence, is an alkali metal. It is silvery-white in appearance and exists in nature in the form of minerals such as sodalite, rock salt, feldspar, etc.